Abstract
INTRODUCTION: Hypomethylating agents (HMAs) are the standard of care of patients (pts) with higher-risk myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML). HMA failure (HMA-F) is associated with poor outcomes, with a median survival of 4 to 6 months. CPX-351 is a dual drug liposomal encapsulation of cytarabine and daunorubicin at a 5:1 molar ratio approved for the treatment of pts with newly-diagnosed therapy-related acute myeloid leukemia (AML) or AML with MDS-related changes. Here we present updated results of a phase 1/2 study of lower dose CPX-351 for high-risk MDS and CMML after HMA-F.
METHODS: We designed a phase 1/2 clinical trial of CPX-351 for pts with MDS or CMML after HMA-F with Int-2 or High risk by IPSS, or Int-1 with >10% bone marrow blasts. The study included an initial phase 1 dose-escalation portion, following a 3+3 design, followed by a phase 2 dose expansion cohort. Dose escalation included 4 dose levels of CPX-351: 10 units/m 2 (daunorubicin 4.4mg/m 2 and cytarabine 10mg/m 2), 25 units/m 2 (daunorubicin 11mg/m 2 and cytarabine 25mg/m 2), 50 units/m 2 (daunorubicin 22mg/m 2 and cytarabine 50mg/m 2) and 75 units/m 2 (daunorubicin 33mg/m 2 and cytarabine 75mg/m 2). Therapy was administered intravenously on days 1, 3 and 5 of 28-day cycles during induction and on days 1 and 3 of re-induction or consolidation. In pts not achieving response after induction, the study allowed re-induction. The primary end point was to evaluate safety and determine the maximum tolerated dose of CPX-351. Responses were evaluated following 2006 IWG criteria. The study included stopping rules for toxicity and futility. The Kaplan-Meir product-limit method was used to estimate median survival.
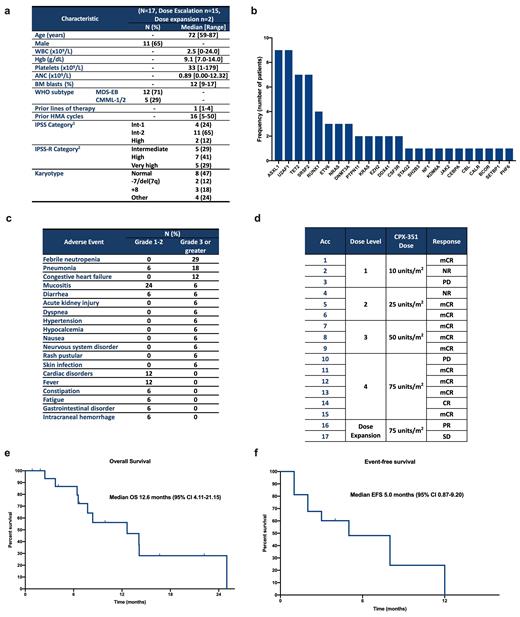
RESULTS: At the current data cut-off of July 27 th 2021 a total of 17 pts have been treated: 15 in the phase 1 portion, and 2 in the phase 2. A total of 12 pts had MDS, and 5 have CMML. Pt characteristics are shown in Figure 1a. Four (24%), 11 (64%) and 2 (11%) pts had Int-1 risk by IPSS with >10%, Int-2 or High risk by IPSS, respectively. The median age was 72 years (range 59-87) and 65% pts were male. Baseline mutations are shown in Figure 1b. The median number of prior therapies was 1 (range 1-4) with a median of 16 (range 5-50) cycles of prior HMA. Three pts had received prior therapy with venetoclax. No DLTs were observed within the 28-day DLT evaluation window of the phase 1 portion. One pt treated at the 75units/m 2 dose level developed congestive heart failure with reduction in ejection fraction after cycle 2 of therapy. An additional 3 pts were included at 75units/m 2 dose level with no DLTs being observed. Enrollment on the phase 2 was initiated at 75units/m 2 with 2 pts treated so far. Overall, the median number of administered cycles of therapy is 2 (range 1-9). A total of 13 (76%) pts had non-hematological adverse events (Figure 1c). Eight-week mortality was 0%. Both pts in the dose-expansion cohort experienced cardiac complications including congestive heart failure without reduction in ejection fraction after cycle 2 of therapy in 1 pt, and right-sided heart failure with fluid overload after cycle 1 in 1 pt. All pts with cardiac events were >75 years of age but none had prior cardiac conditions. The median number of days from the start of cycle 1 to cycle 2 was 49 (range 28-83). Three pts required dose reductions due to cytopenias (2 at 75units/m 2 and 1 at 50units/m 2). All pts are evaluable for response with an overall response rate of 71% (n=12) including 6% CR (n=1), 59% mCR (n=10) and 6% PR (n=1) (Figure 1d). Four pts required re-induction prior to achieving mCR. Median number of cycles to best response was 1 (range 1-3). The median response duration was 2 months (0-10 months). Four pts (24%) proceeded to allogeneic-stem-cell transplantation after achieving response. Two (11%) pts relapsed and 2 (11%) had transformation to AML. With a median follow-up of 16.7 months, the median overall survival is 12.6 months (95% CI 4.11-21.15) (Figure 1e), and the median event-free survival is 5.0 months (95% CI 0.87-9.20) (Figure 1f).
CONCLUSIONS: Preliminary data suggests lower doses of CPX-351 in higher-risk MDS and CMML after HMA-F can induce marrow responses and be used as a bridge to allogeneic stem-cell transplant. In older individuals (>75 years), the dose of 75units/m2 is associated with risk of cardiac toxicity. Enrollment is continuing at a dose of 50units/m 2. Further follow up is required for individuals <75 years of age who are transplant candidates.
Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Kadia: Novartis: Consultancy; Astellas: Other; Liberum: Consultancy; AstraZeneca: Other; Genfleet: Other; Ascentage: Other; Cellonkos: Other; Sanofi-Aventis: Consultancy; Pulmotech: Other; Pfizer: Consultancy, Other; AbbVie: Consultancy, Other: Grant/research support; Aglos: Consultancy; Amgen: Other: Grant/research support; BMS: Other: Grant/research support; Cure: Speakers Bureau; Dalichi Sankyo: Consultancy; Genentech: Consultancy, Other: Grant/research support; Jazz: Consultancy. Ravandi: Novartis: Honoraria; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Honoraria, Research Funding; AstraZeneca: Honoraria; Prelude: Research Funding; Jazz: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; Agios: Honoraria, Research Funding. Faderl: Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Kantarjian: Astra Zeneca: Honoraria; KAHR Medical Ltd: Honoraria; Daiichi-Sankyo: Research Funding; Aptitude Health: Honoraria; Novartis: Honoraria, Research Funding; Ascentage: Research Funding; Precision Biosciences: Honoraria; Immunogen: Research Funding; Astellas Health: Honoraria; Jazz: Research Funding; Amgen: Honoraria, Research Funding; NOVA Research: Honoraria; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Ipsen Pharmaceuticals: Honoraria; Pfizer: Honoraria, Research Funding; Taiho Pharmaceutical Canada: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal